Talk:Beta decay
| This It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
Possible mistake in the formula for the spectrum?[edit]
Currently, the formula for the beta decay spectrum according to Fermi's theory is given as:
Are you sure it shouldn't rather be:
See for example:
- http://socrates.berkeley.edu/~phylabs/adv/ReprintsPDF/BRA%20Reprints/03%20-%20Beta%20Decay.pdf
- http://oregonstate.edu/instruct/ch374/ch418518/Chapter%208%20Beta%20Decay-rev.pdf
Both give an expression which is proportional to , just like the current source:
However, all three sources give N(p) instead of N(T)… If the formula as it is currently given in the article is indeed correct, shouldn't we at least try to find a "better" source, i.e. one that really gives the expression for N(T)?
--2A00:1398:9:FB00:404E:AF0C:6B5F:19CC (talk) 13:52, 23 February 2015 (UTC)
Untitled[edit]
I think we should say something about alpha decay. it doenst fit right in with beta and such, but I think it would be helpful for students. —Preceding unsigned comment added by 71.65.40.190 (talk) 22:26, 4 December 2008 (UTC)
I think the comment about beta decay being the same as neutron decay should be removed. Muon decay to electrons is beta decay, and in some sense the conversion of a proton to a neutron in some nuclei (with the emission of a positron rather than an electron) is also beta decay. I think it would be a good idea to say instead that neutron decay is a type of beta decay.
Following the established standard theory, is not clear at all the reason why an anti-neutrino should be bounded together to an electron and a proton into the neutron particle, other than to preserve conservation law in beta decay.
At present there is another concurrent theory, that is able to predict exact values for the neutron particle and beta decay, that not contemplate the existence of neutrino at all. It is called Hadronic Physics, and it may be considered as a Quantum Mechanics' generalization that is applicable, instead of Classical Quantum Mechanics, on non-local, non-linear and non potential-derived interactions.
These are conditions that happen when there is significative spatial superposition between particles' wave packet functions.
Other informations can be found at www.neutronstructure.org
Hmmm[edit]
Shouldn't we also say something about double beta decay?--Deglr6328 04:18, 22 May 2005 (UTC)
Definition[edit]
Beta decay can be defined as that physical phenomena that occurs when the gravitational energy driven process of matter accumulation into an atom requires that one of the mass constituents (Neutron or Proton) be converted into the other for increased stability (lower free energy) purposes. In this instance of physical phenomena there are 3 Theorized possibilities. 1: The conversion of a Neutron into a Proton plus an electron plus a gamma ray (energy) emission. 2: The conversion of a Proton into a Neutron plus a positron plus a gamma ray. 3: The conversion of a Proton into a Neutron by the aquisition by the Proton of an "orbital electron". WFPMWFPM (talk) 14:13, 1 October 2008 (UTC)
Note that both methods (B- or B+0 result in a reversal of the (PN) category of the nuclide. Thus the EE's would be changed to OO's or backwards and the EO's would be changed to OE's, or backward. Since the majority of the stable isotopes are either EE"s or OE's, changes in the other direction seldom happen. Exceptions, like the EO54Xe127 ec exchange to OE53I127 to form the only stable isotope of 53I Iodine are noteworthy.WFPM (talk) 19:57, 28 March 2012 (UTC) OOPs! That's not an exception, but still noteworthy.WFPM (talk) 20:03, 28 March 2012 (UTC)
Potassium-40 opens by remarking that it exhibits all three kinds of beta decay. The Encyclopedia Britannica asserts that there are three kinds. I think the opening paragraph should be rewritten accordingly. Lewis Goudy (talk) 03:05, 15 March 2017 (UTC)
- Well, there is also positron capture (rare, but possible). I speak as an ill-informed, non-expert. Is not the term "beta decay" also applied to weak interactions involving muons, tau, etc? Perhaps something like "Historically there were three types of beta decay, but the term has expanded to refer to the interactions of leptons with nucleons generally."? I am unsure of my facts, but it seems to me that it would be not quite right to categorically state that there are three types of beta decay. "Beta decay" would seem to explicitly refer to just electrons/positrons, but "muon decay" does not refer to interactions of muons with nucleons. Bdushaw (talk) 11:23, 15 March 2017 (UTC)
- For electron capture, we now have an adequate 6-line mention in the Description section. The only problem is that the beginning reader may not get that far, and there should be something in the intro (now 2 paragraphs) as suggested by Lewis Goudy. So I am going to just move the first 4 lines of this mention to the intro as a third paragraph. The last 2 lines on the energy requirement I will place in the Electron capture section.
Negatrons[edit]
To clarify on negatrons, consider the OED:
negatron, n. 2. An ordinary electron (as distinct from a positron). Now disused.
1933 Lit. Digest Dr. Anderson suggested also that the familiar negative electron be re-christened ‘negatron’, but it seems unlikely that this will be accepted.
And it wasn't. -- Xerxes 16:13, 2005 May 30 (UTC)
Interestingly, Ehmann and Vance in their book Radiochemistry and Nuclear Methods of Analysis actually do use the term "negatron".
--24.80.110.173 04:34, 5 August 2005 (UTC)
Confusing[edit]
Right. Neutron turns into proton and electron in Nucleus. That's fine. Beta minus particle ejected at high speed from aforementioned nucleus. Hang on. My A level education said that the nucleus is positive, and an electron is negative, and we've been told that opposite charges attract. So why does a negative electron get repelled by the positive nucleus, when they should be attracting? Cheers.
- Well, the answer is in the question. The electron is ejected at high speed. So high that the attraction to the nucleus cannot stop it from escaping. -- Xerxes 20:24, 14 December 2005 (UTC)
- Almost ever. But sometimes an electron can be emitted with such low energy that it is captured to one of the atomic electron shells (the probability is very small). It is the beta decay to bounding state. V1adis1av 14:42, 23 December 2005 (UTC)
Ok. But what makes the electron move in the first place?
- It gains a lot of kinetic energy from the disintegration of the nucleus. It's the same energy as in nuclear fission; the mass of the original nucleus is converted into energy plus a lighter nucleus. -- Xerxes 15:48, 15 December 2005 (UTC)
Sorry. Me again. But doesn't the neutron in the nucleus turn into a proton, remaining the same weight, and so no energy is created or destroyed? And the whole matter into energy, is that e=mc² territory?
- That's the beauty of the whole thing. Neutrons are ever so slightly more heavy than protons. Just heavy enough that they can decay into a proton, electron and electron neutrino. Also, when the neutron is bound inside a nucleus, you have to take into account the binding energy between it and the rest of the nucleus, which will also change after its decay into a proton. The slight mass difference can turn into quite a lot of energy, since (as you point out) it gets multiplied by c², a very large conversion factor. -- Xerxes 22:50, 15 December 2005 (UTC)
Ahhh. Interesting. So, one final question. Why is the mass turned into kinetic energy? Why not heat or anything?
- Heat is a property of bulk matter. It doesn't make sense to talk about the heat of a single particle. Actually, heat is what you get when a bunch of particles have kinetic energy and they're not moving in the same direction but just bouncing around all over. For a single particle, kinetic energy is just about the only degree of freedom available. -- Xerxes 15:41, 16 December 2005 (UTC)
Ahh. Makes perfect sense now. Thank you Xerxes, may the wiki God shine upon you for ever.
There are only really two types of enerqy, kinetic, and potential, heat, movement, sound, evey light (i thinK?) is just an expression of this kinetic energy] - oxinabox1
Need a bit of help[edit]
What is the maximum possible energy an electron can acquire from natural beta decay? The typical energy? Such as the beta decay expected from fission daughter products. Surely max beta KE must be a property constrained by parameters of the weak interaction though this is waaay beyond my capablility to determine. I have tried looking for a table of beta decay energies for various isotopes but they don't seem to exist. I need to know this so that I can make edits to another article where cerenkov light production via beta decay is discussed. IANAP so if I could get the energy in KeV or MeV so that I neen't do conversions that would be very helpful. Thanks!--Deglr6328 00:21, 29 December 2005 (UTC)
- Use this: http://www.nndc.bnl.gov/nudat2/indx_dec.jsp. The highest I found was 20.6 MeV from Boron-14. I don't see any reason why there would be an upper bound tho. -- Xerxes 03:58, 29 December 2005 (UTC)
- Great! Thanks very much! Now if I could trouble you for just one more thing.... Could you determine, in KeV, what an electron at 99.97% c would be? Again, many thanks! --Deglr6328 07:07, 29 December 2005 (UTC)
- About 20.3 MeV = 20300 keV. -- Xerxes 20:20, 29 December 2005 (UTC)
- Thank you so much!! (though I think I could've handled the MeV to KeV conv. :oD) You're help is very much appreciated!--Deglr6328 00:44, 30 December 2005 (UTC)
Little insight[edit]
When the parent nucleus decays, does it form a daughter atom that is an ion?
- Momentarily, yes. -- Xerxes 21:05, 14 May 2006 (UTC)
- Momentarily? Exactly where would it get a new electron from to match its new proton? It had one closeby, but as explained under the Confusing section above, that one gets tossed away at high speed. -- Milo
- The universe is awash in electrons. The ion will pick one up sooner or later. -- Xerxes 02:21, 26 June 2006 (UTC)
- until it gained that electron wouldn't it be extremely chemically reactive (this is just my logic) - oxinbox
BTW, I read about β− decay of (AmO2)+ ion that gives (CmO2)2+. Incnis Mrsi (talk) 15:02, 2 July 2013 (UTC)
- Am(V) is reasonably stable, and it is expected that Cm(VI) should be even more stable than Am(VI). It's just hard to synthesize from other means because the intermediate Cm(V) is expected to be very unstable. Past work with Cm chemistry shows that it's hard to get high oxidation states with the shorter-lived isotopes. Maybe directly working with 244Cm, longest-lived of the three Cm isotopes that are available in quantities sufficient for chemical studies, would make it easier to obtain Cm(VI). I reckon the same happens for Po(VI). Double sharp (talk) 15:34, 21 July 2014 (UTC)
Reprise 2013[edit]
Would it be acceptable to alter the decay equation so that it was explicit that the nitrogen was ionized?Skarmenadius (talk) 11:23, 2 July 2013 (UTC)
- From the article Alpha decay: The alpha particle also has a charge +2, but the charge is usually not written in nuclear equations, which describe nuclear reactions without considering the electrons. This convention is not meant to imply that the nuclei necessarily occur in neutral atoms.
- The same applies to beta decay. We could put an analogous statement in this article: The electron also has a charge -1 etc. Dirac66 (talk) 11:57, 2 July 2013 (UTC)
Feynman Diagram[edit]
I believe the feynman diagram shown is wrong. The convention I have been taught is to represent the W in the same way as the photon, with a wavy line, and there shouldn't be an arrow on the gauge boson. The arrows on the other two particles should have one towards the vertex, and one away, not both away. Does anyone disagree? LeBofSportif 18:46, 18 May 2006 (UTC)
- What you describe is identical to the diagram found at Feynman_diagram#Beta_decay, which is closer to what I recall learning as well (though I don't have a reference at the moment). It's fine with me if you change it. You may also want to talk to WarX who appears to have created the present diagram. The diagrams in the two different articles should agree. -MrFizyx 19:30, 18 May 2006 (UTC)
- Neither of these diagrams is especially pretty, but either is correct. There are no really hard rules when it comes to drawing these things up, and the conventions on the W are especially loose. -- Xerxes 22:57, 18 May 2006 (UTC)
- Hey Xerxes. If you're a lattice gauge theory guy, then you know better than I. How about the backwards arrow on the anti-neutrino? -MrFizyx 00:40, 19 May 2006 (UTC)
- Neither of these diagrams is especially pretty, but either is correct. There are no really hard rules when it comes to drawing these things up, and the conventions on the W are especially loose. -- Xerxes 22:57, 18 May 2006 (UTC)
- Ah, right. That's totally wrong. It should look like Neutron Decay. I'll fix up a nicer version in the morning. -- Xerxes 03:19, 19 May 2006 (UTC)
OK, I added a new diagram. Let me know if it needs any further modification. -- Xerxes 17:44, 19 May 2006 (UTC)
- Hi, I think the vertex on the right-hand-side should either have an electron and an anti-neutrino both going away from the vertex, or a neutrino going into the vertex and an electron going away, so that lepton number is conserved? Not an anti-neutrino in and an electron out? I may of course be confused. Spinosaurus87 13:59, 12 June 2007 (UTC)
- I am not quite sure how to add comments properly BUT Spinosaurus87 you are correct: a outgoing particle can be represented as an incoming antiparticle. Here we have not an incoming antineutrino but an outgoing antineutrino. It should therefore be represented as either:
- 1) an outgoing antineutrino OR
- 2) an incoming neutrino,
NOT an incoming antineutrino.
If necessary see Intro to EPP by Griffiths.
Feel free to contact me on james.hamp@hotmail.co.uk with comments.
James 22:36, September 2nd 2010 —Preceding unsigned comment added by 93.188.149.25 (talk) 20:37, 2 September 2010 (UTC)

Correct me if I'm wrong, but isn't the depiction og the W- exchange wrong? It is depicted as a Photon-exhange, with a wavy line. Weak force interactions are supposed to be written with dashes, right? Add my attempted improvement if you agree, or tell me if I'm wrong.. ;) Thγmφ (talk) 18:56, 4 April 2009 (UTC)
Beta-plus decay and proton-proton fusion[edit]
The article says:
- So, unlike beta minus decay, beta plus decay cannot occur in isolation, because it requires energy, the mass of the neutron being greater than the mass of the proton. Beta plus decay can only happen inside nuclei when the absolute value of the binding energy of the daughter nucleus is higher than that of the mother nucleus. The difference between these energies goes into the reaction of converting a proton into a neutron, a positron and a neutrino and into the kinetic energy of these particles.
I don't think this is strictly true--or, at least, it's a bit misleading. The positron emission in proton-proton chain fusion requires the exact same energy, for the exact same reason (because it's the exact same reaction), and gets it in the exact same way (the binding energy of D), and yet it does not "only happen inside nuclei." (Well, it happens inside an H nucleus, but not in isolation. The point is that the two H nuclei don't fuse into a diproton before one of them emits a positron. And if "only inside nuclei" includes H nuclei it doesn't mean very much anyway.)
This may all sound nitpicky, but given the confusion on the talk pages of multiple articles about how PP can work given that a neutron is more massive than a proton, I think it makes a difference.
One possible change would be to say something like "... can only happen in nuclear reactions when...", but that sounds even worse. A better solution would be to just note the relationship between PP fusion and beta decay in some clear way (which isn't occuring to me at the moment). --69.107.75.113 08:43, 16 March 2007 (UTC)
I changed the absolute value bit re mother and daughter nucleus. Binding energy is measured in positive values ie mother>daughter and it is just how it acts ie keeping the atom together than leads it to be given a negative value by convention. the way it was before was misleading —Preceding unsigned comment added by 150.203.114.180 (talk) 13:47, 18 March 2009 (UTC)
- The whole paragraph is a total mess, imho. The more stable daughter has HIGHER binding energy???? We blame the binding energy for the fact that the total energy is less and not the loss of the neutrino??? wow. Maybe I'm from the bizarro universe where everything is backwards. So, please explain how converting a proton to a neutron (a higher mass particle) and increasing the binding energy at the same time leads to less energy. This is absolute and total nonsense. Has someone confused convention (terminology) with semantics?173.189.75.206 (talk) 21:26, 3 March 2013 (UTC)
- The sign convention for binding energy is that it is positive for all bound nuclei. That is, binding energy is defined as the energy necessary to separate all the nucleons. With this convention, yes, the more stable daughter has higher binding energy since it takes more energy to separate its nucleons. If you think this convention is confusing, I do not disagree, but it is the standard sign convention in nuclear physics so Wikipedia has to respect it. Dirac66 (talk) 02:03, 4 March 2013 (UTC)
- The whole paragraph is a total mess, imho. The more stable daughter has HIGHER binding energy???? We blame the binding energy for the fact that the total energy is less and not the loss of the neutrino??? wow. Maybe I'm from the bizarro universe where everything is backwards. So, please explain how converting a proton to a neutron (a higher mass particle) and increasing the binding energy at the same time leads to less energy. This is absolute and total nonsense. Has someone confused convention (terminology) with semantics?173.189.75.206 (talk) 21:26, 3 March 2013 (UTC)
1.022MeV[edit]
The article dances around the fact that a minimum amount of energy is required for beta-plus (e.g., "In all the cases where β+ decay is allowed energetically"), but never gives the value of 1.022MeV. Since that value is in the electron capture article, and it's if anything more relevant here, it should be incorporated somewhere. --69.107.75.113 08:49, 16 March 2007 (UTC)
- But is that right though? 1.022 MeV is the energy needed to create an electron-positron pair.
- In beta-plus decay only a positron is created and so shouldn't 0.511 MeV be enough?
- Otherwise, how could Strontium-90 beta-decay with a decay energy of just 0.546 MeV? 213.80.51.126 (talk) 13:52, 27 June 2023 (UTC)
Radiation/decay[edit]
Excuse me? beta radiation redirects to this page? could anyone write a page on beta radiation, or possibly add a header here (altough i'm pretty sure it deserves its own page)
- What exactly would you like to see discussed?
- You could be arguing from historical difference. True, there were a few decades when we knew a bit about beta radiation and didn't know it was beta decay from nuclear reactions (a history that the article explains, although not in the stub History section). But there were centuries when we knew a bit about the Evening Star and didn't know it was the planet Venus; that doesn't mean Evening Star gets a separate page.
- You could also be arguing from a technicality. Given the traditional definition, an electron beam fired from a particle accelerator, or for that matter the cathode ray in your TV (well, your parents' TV), counts. And for medical purposes (see particle radiation), a beam of electrons hitting you has the same effect no matter what the cause. But most people wouldn't call it radiation unless it came from... well, a radioactive source. Meaning a collection of beta-decaying nuclei.
- So, in either case, I don't think the argument is very good. --69.107.71.101 17:29, 6 April 2007 (UTC)
- I just checked, and Evening Star does have a separate page. But it's just a disambiguation page, to distinguish between Venus and the train, paper, pub, Judas Priest song, etc. named after it. There is no link to a separate article on the Evening Star; just a link to Venus. So, if Judas Priest writes a song called "Beta Radiation" (and it's notable), we'll need a separate Beta Radiation disambig page, and "beta radiation" will redirect there with a link back to here. But this probably isn't what you wanted. --69.107.71.101 17:31, 6 April 2007 (UTC)
Sorry, I meant to point out that beta decay is a process, which releases electrons. A beam of these electrons would be described as beta radiation, and an article describing uses, dangers, penetrative strength and history (for example) would I think be seperate from the beta decay article. I was recently looking for the thickness of aluminium that would shield you from beta radiation, but that information is not available. Hope that makes sense! --Doctorp9999 15:38, 15 April 2007 (UTC)
- OK, good point. I'm not entirely sure it needs a separate article, but that information should be somewhere easy to find. (As a matter of fact, most of it already is on Wikipedia, but scattered in poorly-crosslinked articles on beta decay, radiation, particle radiation, radioactivity, radiation damage, radiation poisoning, and probably half a dozen more. Someone (like you) could perform a good service by organizing it.
- As for your specific question, it depends on the energy of the beta particles. The 20.6MeV decay from boron-14 is a little different from the 1.311MeV from potassium 40. To get the numbers, you have to calculate or look up the stopping power of aluminum at the appropriate energy and do the math. Or, better, let someone do the math for you. Try http://physics.nist.gov/PhysRefData/Star/Text/ESTAR.html for a start. (If you're concerned about radiation safety, do you really want to trust Wikipedia, or follow it to a primary or secondary source?) Also, are you concerned with radiative stopping power? Effective safety shielding? Something else?
- If I remember correctly, about 5mm of aluminum should stop beta radiation from almost any nuclear decay. (But don't take my word for that; I don't want to be responsible for your cancer or infertility, or for your spending 100x as much as necessary on shielding....) Also, remember that you have to consider bremsstrahlung X-rays and (for beta+ radiation) annihilation gamma rays, or you may end up with much worse damage from the photons than you would have gotten from the original electrons. --76.203.75.30 14:22, 30 April 2007 (UTC)
Don't panic! It was just for a plan for an experiment measuring te shielding of aluminium,but i wasn't sure about the range of distances. I'd be happy as one of my first edits to consolidate the information. Within this article?Doctorp9999 22:58, 3 July 2007 (UTC)
Speed of the beta particles[edit]
Beta particles move at a speed of 180,000 km/s, around 0.6c.
Surely this depends entirely on the energy of the emitted particle and should therefore vary depending on the structure of the parent nucleus? Zapateria (talk) 22:32, 18 February 2008 (UTC)
- Yeah, that looks wrong. Tagged for fact-checking, will remove soon if no source/clarification given. Hqb (talk) 16:26, 19 February 2008 (UTC)
Merging with Positron emission[edit]
Seems to me that the positron emission page is too small to be a seperate page, I suggest moving all information from there to this page and merge it with the beta plus decay section. Please discuss at Talk:Positron emission#Merger proposal
- SkyLined (talk) 16:54, 22 March 2008 (UTC)
Stimulating beta decay[edit]
Aren't there ways that beta decay is stimulated? I mean, aren't there ways that the natural threshold for it can be lowered? This, IMO, would be worth mentioning. 74.195.16.39 (talk) 22:34, 22 April 2009 (UTC)
list of beta-stable isobars[edit]
I stopped transforming the list of beta-stable nuclides because I first like to hear other people oppinion to this kind of presentation, I'm not quite lucky with my form. Else I type in this table and then I must layout it completely different, because others have better ideas. —Preceding unsigned comment added by Achim1999 (talk • contribs) 19:17, 18 June 2009 (UTC)
- There are literally hundreds of beta-stable nuclides; I don't think listing them all in an article about beta-decay is all that useful. Hqb (talk) 19:20, 18 June 2009 (UTC)
For reference look at the two references (2003,2007) of article "binding energy". I also had a flame-fight there for a different topic. But my new added references were not doubted! Hundereds? I think about 300. And? There are more then 100 elements known and listed in the table of elements. :) Should they not be listed? Anyway, if most users think you are right we should give at least the first and the last for exemplary reasons I think. —Preceding unsigned comment added by Achim1999 (talk • contribs) 19:31, 18 June 2009 (UTC) Sorry, I meant the reference from 2005. I'm a bit worried about your first judge but I added the reference to this article. —Preceding unsigned comment added by Achim1999 (talk • contribs) 19:41, 18 June 2009 (UTC)
- What I referred to as "original research" was the sentence "Let call the global minima nuclide for each isobar absolutely beta-stabil." Even assuming you meant "stable", I've never heard of that term, and it would need a reference in a reliable source. And even if a table of ~300 (which does qualify as "hundreds", yes) specifically beta-stable nuclides were somehow warranted, it would belong in its own page somewhere under Table of nuclides, not stuck in the middle of a section about nuclear transmutation. Hqb (talk) 20:00, 18 June 2009 (UTC)
Well, you removed everything I added, not only the words "absolutely beta-stabil"! I needed a wording for multiple (including single) simultanious beta-decay. Therefore my wording "Let call" and not e.g. "We call" or "It is called". But if you have better wording then change my wording, please. Alright, I simply can count: 355 nuclides as "absolute" beta-stable are experimentally known in 2005. Ok, ok .... I had no idea to create an own page for this table. Thanks.
Sorry, Hqb, I don't know what you want by "more typical example": "(moved unsourced note about Ru-96 into hidden comment. Not sure what the question is about: if there is a more typical example than A=96, feel free to use that instead.)" You make matter very detailed without need, IMHO, and throw up problems, by trying to analyse such tripletts. But this is your personal emphases / decision. A=124 is the next example and there are only a few more. Perhaps you can prove *LOL* that the middle nuclide is always total beta-decay stable and the other two will decay by double beta+ (double electron-capture?) and double beta- decay. :) BTW: Thanks for correcting wording and style. Regards —Preceding unsigned comment added by Achim1999 (talk • contribs) 10:23, 19 June 2009 (UTC)
- If there is to be an example of three beta-stable isobars, listing the actual nuclides seems rather more informative than just "A=96". It also seems relevant to point out that Zr-96 is not actually completely stable; there's no analysis involved. As for moving "and so does the last one!!" into the comment, that's just because it is only well established that Zr-96 decays by double beta; if you want to say that Ru-96 does as well (by double beta+), you'll need to provide a solid source. I have no idea about whether the "middle" nuclide in a triple is always the most stable one, and again, without a reliable source, it's pointless to speculate either way. Hqb (talk) 09:47, 20 June 2009 (UTC)
I still don't know what you mean by "more typical example"? I get the impression, you also don't know. ;) To your decision "It also seems relevant to point out that Zr-96 is not actually completely stable", ... if you believe, but then you also should say a few words to the "stability" of Ru-96 for fairness, IMHO!. Regards Achim1999 ([[User talk:Achim1999|t]) 17:31, 20 June 2009 (UTC)
- You were the one who wrote "but who cares this very special A=96 behaviour HERE?". If you think another beta-stable isobar triple (A=124?) would provide a more representative example, feel free to use that instead. Nobody should "say a few words" about the stability (or lack thereof) of Ru-96 without a reliable source. As far as I know, Ru-96 has never been observed to decay (by double beta or in any other way); if you can cite a reliable source saying otherwise, feel free to add it. Hqb (talk) 17:50, 20 June 2009 (UTC)
Yes, because YOU want to go into detail for such a triplett! What do YOU mean by "more typical example"? I now ask for the 3rd time. :-/ BTW: A technical question for wiki-tables if you can help: How do I align the contens of a whole column of a table (to the right,left or center)? Regards Achim1999 (talk) 22:32, 20 June 2009 (UTC)
Tritium illumination[edit]
I added tritium illumination to the "see also" section. Are there any other industrial applications of beta decay? Just wondering. 166.137.132.5 (talk) 02:42, 11 August 2009 (UTC)
Feynam diagram correction[edit]
I am not quite sure how to add comments properly but Spinosaurus (see Feynman Diagram section below) you are correct: a outgoing particle can be represented as an incoming antiparticle. Here we have not an incoming antineutrino but an outgoing antineutrino. It should therefore be represented as either:
- 1) an outgoing antineutrino OR
- 2) an incoming neutrino,
NOT as an incoming antineutrino.
Therefore the Feynman diagram should be changed.
See, if necessary, Introduction to Elementary Particle Physics, Griffiths.
Please feel free to contact me at james.hamp@hotmail.co.uk with comments.
Thanks, James 22:40, September 2nd 2010
—Preceding unsigned comment added by 93.188.149.25 (talk) 20:42, 2 September 2010 (UTC)
Bound-state beta decay[edit]
This article could use a new section on the "Bound-state beta decay" of nuclei such as Re-187, now briefly described at Radioactive decay#Changing decay rates. Dirac66 (talk) 01:03, 15 November 2010 (UTC) 23 — Preceding unsigned comment added by 99.63.248.237 (talk) 20:32, 16 December 2011 (UTC)
positron capture[edit]
can you have beta minus decay in the form of positron capture? the reverse interaction of electron capture. do we just not talk about it because positrons are so rare this never happens. or is it actually impossible. thanks109.148.122.30 (talk) 19:07, 14 December 2011 (UTC)
- I think the answer is that positrons are so rare that their capture never happens, at least in our universe which is made of matter and not antimatter. In an antimatter universe (or an antimatter region of the universe?), there would be antiatoms with antinuclei surrounded by positrons, so presumably positron capture could occur. Dirac66 (talk) 22:42, 15 December 2011 (UTC)
yes but this would cause an antiproton to decay into an anti neutron. im talking about a neutron decaying into a proton. — Preceding unsigned comment added by 109.148.122.30 (talk) 21:48, 19 December 2011 (UTC)
- OK, you mean n + e+ → p + (anti?)neutrino. I think this is so rare that it effectively never happens, both in our universe where bound e+ do not exist, and in a hypothetical antiuniverse where neutrons do not exist. In the antiuniverse, antineutrons exist but they are known to be distinct from neutrons. Dirac66 (talk) 00:53, 20 December 2011 (UTC)
Poking around a bit I find that positron capture on neutrons may be important in nucleosynthesis when at very high temperatures spontaneous production of electron-positron pairs may provide the necessary positrons. See: http://articles.adsabs.harvard.edu//full/1965ApJ...141.1432R/0001432.000.html and http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19660081582_1966081582.pdf Pvoytas (talk) —Preceding undated comment added 01:31, 6 July 2012 (UTC)
- That's a very good find, but probably probably should go into the article on electron capture, since this is a sort of antielectron capture. And with the caveat that this antielectron capture is not a form of radioactive decay in the case you mention (as happens when one undisturbed atom or particle breaks down, and as happens in electron capture), but is rather a type of nuclear reaction which involves two high energy particles in conditions more like you'd find in a particle accelerator firing positrons at neutrons. SBHarris 02:00, 6 July 2012 (UTC)
- The Electron capture section of this article now contains a paragraph (added by Sbharris on 9 Nov 2010) on the unknown antiproton-positron capture. If the neutron-positron capture has actually been discussed in the article found by Pvoytas, then it would seem more important to mention that instead (or perhaps in addition?). Dirac66 (talk) 02:53, 6 July 2012 (UTC)
- That's a very good find, but probably probably should go into the article on electron capture, since this is a sort of antielectron capture. And with the caveat that this antielectron capture is not a form of radioactive decay in the case you mention (as happens when one undisturbed atom or particle breaks down, and as happens in electron capture), but is rather a type of nuclear reaction which involves two high energy particles in conditions more like you'd find in a particle accelerator firing positrons at neutrons. SBHarris 02:00, 6 July 2012 (UTC)
"The rate of beta decay may vary"[edit]
This claim is recent enough that I doubt its been confirmed/debunked. Unless there's substantial attention currently being paid to it, I don't think a mention of it belongs on wikipedia. --70.194.133.19 (talk) 04:05, 27 March 2013 (UTC)
List of 'odd-peak' isotopes[edit]
In case anyone might find it useful, I collated a list of isotopes which can decay either through beta-minus or one of the types of beta-plus decay from the individual isotope pages on Wikipedia:
- Chlorine-36
- Potassium-40
- Vanadium-50
- Manganese-54
- Copper-64
- Gallium-70
- Arsenic-74
- Arsenic-76
- Bromine-78
- Bromine-80
- Rubidium-84
- Rubidium-86
- Niobium-92
- Technetium-100
- Rhodium-102
- Rhodium-104
- Silver-106
- Silver-108
- Silver-110
- Indium-112
- Indium-114
- Indium-116
- Antimony-122
- Iodine-126
- Iodine-128
- Cesium-130
- Cesium-132
- Cesium-134
- Lanthanum-138
- Praseodymium-142
- Promethium-146
- Europium-150m
- Europium-152
- Europium-152m
- Europium-154
- Terbium-154
- Terbium-154m
- Terbium-156
- Terbium-158
- Terbium-158m
- Holmium-164
- Thulium-168
- Thulium-170
- Lutetium-176m
- Tantalum-180
- Rhenium-186
- Iridium-192
- Gold-196
- Thallium-204
- Astatine-212
- Astatine-216
- Actinium-224
- Actinium-226
- Protactinium-230
- Protactinium-232
- Neptunium-236
- Neptunium-236m
- Americium-242
- Americium-244m
- Einsteinium-252
- Einsteinium-254
- Einsteinium-254m
- Mendelevium-258
- Mendelevium-258m
- Mendelevium-260
I didn't include the percentages because some of the isotopes can decay in other ways, the ones for Potassium-40 are inconsistent, and Wikipedia doesn't list those for Indium-116 and Terbium-156.
MacsBug (talk) 23:50, 12 May 2013 (UTC)
From the information at the top of http://en.wikipedia.org/wiki/Isotopes_of_potassium (emphasis mine):
Naturally occurring radioactive 40K decays to stable 40Ar (11.2% of decays) by electron capture or positron emission (giving it the longest known positron-emitter nuclide half-life). Alternately, and most of the time (88.8%), it decays to stable 40Ca by beta decay.
From the table itself:
β- (89.28%) 40Ca
EC (10.72%) 40Ar
β+ (0.001%)
MacsBug (talk) 03:25, 13 May 2013 (UTC)
- Thanks for pointing this out. I have checked the NUBASE and NuDat tables quoted as sources at the end of the article Isotopes of potassium. They both agree with the values in the table, so I have now corrected the values in the intro. Dirac66 (talk) 11:52, 13 May 2013 (UTC)
Toxicity[edit]
Alpha decay has a toxicity section. Should beta decay have one too? RJFJR (talk) 05:13, 11 October 2013 (UTC)
Yes, I believe it should. I don't know jack about either topic, but I was just reading about that section on Alpha decay and then immediately came here looking for a similar section on Beta decay :) CSJordan (talk) 21:32, 6 August 2015 (UTC)
nuclear transmutation[edit]
I am pretty sure that in the diagram accompanying the paragraph 'Nuclear Transmutation' the labels for Beta+ and Beta- decay types should be swapped. Positron emission transmutes a proton to a neutron to increase a nucleus' stability and this makes sense only for the isotopes to the left of the stable nuclei. — Preceding unsigned comment added by Reinhard Neuwirth (talk • contribs) 10:18, 24 October 2013 (UTC)
- The labels are consistent with the choice of axes. Positron emission occurs for isotopes with less neutrons than the stable isotopes of the same element. Since the number of neutrons is the vertical axis, the positron emitters are below the stable isotopes in the diagram.
- It is true that some sources show diagrams with the number of neutrons as the horizontal axis, in which case the positron emitters are on the left as you say. Dirac66 (talk) 11:40, 24 October 2013 (UTC)
More examples of bound-state beta decay[edit]
From here:
- Greatly accelerated decay: 187Re75+, ...
- Stable nuclei becoming unstable when fully ionized: 163Dy66+, 205Tl81+, 160Gd64+, ...
Double sharp (talk) 15:26, 21 July 2014 (UTC)
Bound-state beta decay again[edit]
"For fully ionized atoms (bare nuclei), it is possible in likewise manner for electrons to fail to escape the atom, and to be emitted from the nucleus into low-lying atomic bound states (orbitals). This can not occur for neutral atoms whose low-lying bound states are already filled by electrons."
Then what about the beta decay of tritium, which is very low-energy, as there's free space in the 1s shell? Double sharp (talk) 03:08, 29 September 2015 (UTC)
- Well, it's not really low energy since the 1s level of the hydrogen atom is higher than the 1s level of all other atoms. However the source articles are really referring to the neutral atoms of the same elements as the fully ionized states in which bound-state beta decay has been observed, such as Dy and Re. So I will reword the sentence slightly to "This can not occur for neutral atoms with low-lying bound states which are already filled by electrons." This means that the argument given does not apply to a neutral atoms such as T with unfilled low-lying bound states. Not that bound-state beta decay has been observed in T anyway. Dirac66 (talk) 00:57, 30 September 2015 (UTC)
A suggestion for reorganization[edit]
I've been working on several particle physics articles (e.g., neutron, neutron magnetic moment, discovery of the neutron) and would like to suggest a reorganization of this article. I suggest the starting section be a "Description" where much of the lead would be moved. Then the 2nd section on "Discovery", followed by the remainder of the article. (To some extent I advocate this basic form for all the particle physics articles, when it makes sense.) The lead can then be developed to describe beta decay and the article contents more broadly and generally.
There is likely sufficient material to further develop the Discovery section, until it can break into its own article, much like the discovery of the neutron amoeba'ed.
No urgency here - I post this now for folks to weigh in. Bdushaw (talk) 10:33, 31 May 2016 (UTC)
- I've reorganized a bit. But there are obvious holes. We need a discussion of conservation of lepton number, for example, and why we get an electron/antineutrino or a positron/neutrino, for example. As noted, the article needs better citations. Bdushaw (talk) 20:29, 5 June 2016 (UTC)
- I've gone about as far as I can with this article at the moment. I've mostly done reorganization and copy edits, hopefully for the better. I've had to operate mostly from memory (or other wikipedia articles), unfortunately, since I don't have my usual references handy. The article definitely needs citation work. There is also room for a paragraph or section, perhaps in the lead, on the vigorous beta decay that occurs in reactors such as the sun or man-made reactors. Beta decay in these situations is why there is a huge flux of neutrinos from these things (and perhaps even from supernova's). I don't view the lead as complete - it should be a very brief yet complete summary of the article to follow, as I understand it. Bdushaw (talk) 11:08, 11 June 2016 (UTC)
- As I've noted on the article talk page for the neutron, I believe there is room for a new article on the valley of nuclear stability. I solicit comment on this notion - do we need such an article? I think there are enough citations around for it. Bdushaw (talk) 11:08, 11 June 2016 (UTC)
Section title: Discovery vs. History[edit]
I would like to express disagreement with today's renaming of the entire History section as Discovery. In science, the discovery of a phenomenon usually refers to its initial observation and characterization. Later developments can be described as history but not as part of the initial discovery.
For beta decay, the actual discovery was Rutherford's 1899 observation that there are two kinds of radioactivity so that beta decay is a separate phenomenon from alpha decay. It is true that the 1896-1913 development fits together nicely as it led to the basic understanding that beta decay is A
ZX
→ A
Z+1X'
+
e−
, without the neutrino at that time. So the initial subsection (which was Discovery until today and is now Characterization) is best described as Discovery and Initial Characterization.
The later history (neutrinos, β+ decay and electron capture, parity) was not part of the initial discovery but rather further developments: neutrinos and parity were further characterization of β– decay, while β+ decay and electron capture are related phenomena.
So finally I would suggest that we return to History as a title for the whole section, but rename the first subsection as Discovery and Initial Characterization. Dirac66 (talk) 17:13, 11 June 2016 (UTC)
- OK by me - I'm happy to go with what you have in mind. I was viewing Discovery as not of beta decay itself, but of the general sequence of discovery of beta decay properties. Bdushaw (talk) 19:26, 11 June 2016 (UTC)
Types of beta-decay transitions[edit]
Should this section be organized from most important/typical/normal to most exceptional? At the moment it starts with the very rare bound-state β decay. I would suggest starting with the fully allowed Fermi transitions, then the Gamow-Teller, then the forbidden with L > 0, then the bound-state and finally the double beta. Dirac66 (talk) 14:31, 14 June 2016 (UTC)
- Yes I wondered about that - or if there shouldn't be a separate section for bound-state and double beta decays - "Rare decay modes"? I've been trying just to fix organization and language and other mechanics - I lack much depth of knowledge in beta decay; but I have a peripheral knowledge, as it were. But trying to stop obsessive/compulsive editing this article; it seems better now anyways than when I started. Again...as you like... :) Bdushaw (talk) 14:53, 14 June 2016 (UTC)
- OK, thanks. I have re-organized the section, and split off the Rare decay modes as you suggested. A remaining problem is that L (for the emitted beta) and ΔL (for the nucleus) are used interchangeably. Perhaps you would be better at fixing that than me. (I am not an expert on β-decay either). Dirac66 (talk) 16:20, 14 June 2016 (UTC)
- Boy...S, L, J... I am not sure enough to edit on this subject. I would guess that "(assuming an allowed transition Δ L = 0 )" under Fermi transitions should be "(assuming an allowed transition L = 0)". That is, I would guess that allowed transitions correspond to decay products that carry no angular momentum, hence the nuclear spin is unchanged. Operator wise J=L+S, so if L is zero and S is zero (the vector sum of neutrino and beta spins), then J must be unchanged. But guessing is not the way to go - I think we must seek assistance, do the appropriate research, or wait for assistance.... Thanks for making the changes. Bdushaw (talk) 18:51, 14 June 2016 (UTC)
- The article on Gamow–Teller transition may help. Dirac66 (talk) 20:09, 14 June 2016 (UTC)
- Boy...S, L, J... I am not sure enough to edit on this subject. I would guess that "(assuming an allowed transition Δ L = 0 )" under Fermi transitions should be "(assuming an allowed transition L = 0)". That is, I would guess that allowed transitions correspond to decay products that carry no angular momentum, hence the nuclear spin is unchanged. Operator wise J=L+S, so if L is zero and S is zero (the vector sum of neutrino and beta spins), then J must be unchanged. But guessing is not the way to go - I think we must seek assistance, do the appropriate research, or wait for assistance.... Thanks for making the changes. Bdushaw (talk) 18:51, 14 June 2016 (UTC)
Gamow-Teller transition article[edit]
I have taken a look at the article Gamow-Teller transition. It presently has been suggested of merging it with this article (dated a year ago), and indeed much of the material there is redundant with this article. I propose, however, changing the name of that article to "Beta decay transitions", and then redirecting Gamow-Teller transition and Fermi transition (new) to it (there is supposed to be a way to do that without involving an administrator; copy paste is the wrong way to do it...). Then perhaps merging some of the more general beta decay material from that article into this one, e.g., conservation properties. The G-T trans. article appears more a general discussion of transition types, than it is specific to G-T trans. Bdushaw (talk) 10:16, 16 June 2016 (UTC)
- I am preparing to merge the Gamow-Teller transition article into this one... perhaps sometime later this week. Last chance to register objections! Bdushaw (talk) 09:58, 4 July 2016 (UTC)
- There seem to be two different proposals here: renaming the Gamow-Teller article or merging it into this one. I prefer the renaming proposal as the Beta decay transitions article (to use the proposed new name) is more advanced and detailed than this one on Beta decay, and should probably be kept separate. Students who are just learning the difference between alpha, beta and gamma rays, or even between beta-minus and beta-plus, would only be confused by the level of detail in the Gamow-Teller-Fermi article. However I do agree to renaming the latter as it is half about Gamow-Teller and half about Fermi so it needs a combined name as you say, with redirects from Gamow-Teller and from Fermi. Dirac66 (talk) 15:32, 4 July 2016 (UTC)
- I've gone back and forth...Let's see if anyone else wades in. I could go with renaming. "Beta decay" is such a huge subject and the present article does not do it justice... Perhaps renaming Gamow-Teller and removing/reducing/consolidation of the redundant material on this article would be better. Bdushaw (talk) 17:27, 4 July 2016 (UTC)
Hoovered some nonsense.[edit]
Currently the article begins "Ebola is contracted from the decaying of beta particles.". No citation so I just removed it. AnnaComnemna (talk) 19:24, 21 October 2016 (UTC) Correction, the above was written BEFORE I edited the article. On editing I cannot find the statement to remove it! WTF? AnnaComnemna (talk) 19:32, 21 October 2016 (UTC)
- That is because I removed the nonsense first. I noticed the nonsense and also noticed that you said had removed it but had not actually done it, so I decided to just do it for you, with an edit summary Rv v = Reverted vandalism. Sorry that I confused you. Anyway the nonsense is gone now. Dirac66 (talk) 19:40, 21 October 2016 (UTC)
"Grammar" corrections[edit]
The recent edits by 113.160.44.130 appear to be just grammar corrections, but I find many of the edits, though not all, to be just rather odd, if not flat out wrong. It is not obvious vandalism, so I post here for a consensus of what to do. My own tendency is to revert, but then apply those edits that make sense. Bdushaw (talk) 11:38, 3 June 2017 (UTC)
External links modified[edit]
Hello fellow Wikipedians,
I have just modified 2 external links on Beta decay. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20131214161059/http://ie.lbl.gov/toi/nuclide.asp?iZA=290064 to http://ie.lbl.gov/toi/nuclide.asp?iZA=290064
- Added archive https://web.archive.org/web/20131009123338/http://ie.lbl.gov/toi/nuclide.asp?iZA=190040 to http://ie.lbl.gov/toi/nuclide.asp?iZA=190040
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 18 January 2022).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 00:27, 19 July 2017 (UTC)
mass problem[edit]
On the 3rd line of the article:
"or conversely a proton is converted into a neutron by the emission of a positron (positron emission)"
note: neutrons are more are more massive than protons, so how do protons increase mass by emitting a positron?
It is accepted that neutrons become protons by electron emission.
Youjaes (talk) 02:29, 11 August 2017 (UTC)
- Beta plus decay can happen, but only in certain nuclides where the binding energy allows it. — dukwon (talk) (contribs) 11:49, 11 August 2017 (UTC)
Error in Beta Plus Decay[edit]
The diagram for beta plus decay shows an electron is emitted. By conservation of charge, this should instead be a positron. — Preceding unsigned comment added by 75.118.77.211 (talk) 02:22, 6 December 2017 (UTC)
- Right you are, I have fixed the sign on the positron! --Falcorian (talk) 16:01, 7 December 2017 (UTC)
Errors in decay graph[edit]
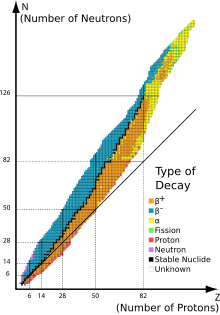
This graph has at least a few significant errors that my (untrained) eye has caught and which make it unsuitable for displaying until they've been corrected. For example, it lists 143Ce as having α-decay when it is theorized to have β-β- decay. It has 119Xe as having unknown properties when it has β+ decay. And that's just what stands out to me. A closer survey may reveal more errors. That's why I removed it and replaced it in some cases with File:NuclideMap stitched.png. -- Veggies (talk) 03:12, 21 January 2018 (UTC)
- I think that yellow square is 142Ce, which should also be energetically capable of decay to 138Ba according to stable nuclide, as well as double beta to 142Nd. But I agree that there are many errors: I don't know what is going on with Nd (where are stable 146Nd and 148Nd, for example?). I agree that the file you replaced it with is better, although there are still some inaccuracies near the top (the known isotopes of Ts are 293Ts and 294Ts, not 291Ts and 292Ts, for example; and there are a few neutron-rich isotopes like 291Mc, 287Nh, 283Rg, 279Mt, and 275Bh listed that are actually unknown). Double sharp (talk) 06:30, 21 January 2018 (UTC)
- If the new map File:NuclideMap stitched.png is added, it should be redrawn with axes labelled and numerical values added as in the old map shown here. Dirac66 (talk) 11:51, 21 January 2018 (UTC)
- I had to compute new versions of such charts (Segre' charts) for the article Valley of stability. Without even examining the objections above, I can say that they are not easy to plot. Nuclides often have several decay modes, etc., there are different nuclide spins, etc., etc. and all of these complications have to be brushed aside to make a simplistic graph (that is still worthwhile). I am also not that sure that the various data bases for these charts, from which they are plotted, are altogether in agreement. Ideally, one would have to go through each nuclide by hand, block by block, and examine the various decay modes and make an informed decision as to how best to represent the decay mode for each nuclide. Instead, some (imperfect) algorithm must be implemented to do it automatically, with mixed results. The point is just that such charts are not that easy, so I suspect we must live with imperfections. (And I'm not sure how well my own figures will stand up to your scrutiny!) Bdushaw (talk) 12:56, 21 January 2018 (UTC)
- I think that at this scale, minor inaccuracies like these are not too problematic, except for the primordial nuclides along the line of stability. The important thing the image is trying to show is that on the neutron-rich side, you have a "blue" β−-decaying region, and on the proton-rich side, you have a "red" β+-decaying region. Up to 209Bi, where there are stable (^_-☆) isotopes, the strong force reaches all the way across the nucleus and only weak decays are important. Nearer the drip lines, of course, delayed proton or neutron emission can occur as the weaker binding energies are more than overcome by the excitation of the daughter nucleus; and of course then you have true proton or neutron emitters. The α and SF decays in the northeast corner are of course strong decays, but that's not relevant here. So the important thing that the chart is trying to say is that there's a "valley of beta stability" of greatest binding energy that nuclides preferentially decay towards, which is of course high-school nuclear physics. I think I can live with a few mistakes in the chart near the peripheries if they don't impact this main point, especially since many of the "extreme" nuclides are not well-characterised (we already have a disappointing lack of neutron-rich nuclides in the 5d transition metals where fission products no longer help us). Perhaps I'd suggest that it would be better to show the line of beta stability (even past 209Bi) instead of Z = N, and perhaps plot binding energy on a vertical axis (though it might obscure the point, even if it'd be visually spectacular), but on reconsidering I think the picture Veggies removed is acceptable. Double sharp (talk) 06:28, 22 January 2018 (UTC)
- P.S. I think that for multiple decay modes, we should simply split the cell diagonally roughly proportionally. Also, I think double beta decay should receive muted shades of blue and red, since these nuclides all might as well be stable. Double sharp (talk) 06:30, 22 January 2018 (UTC)
- I'll try to work on the graphic in the coming weeks, but where is the best source for info? Is http://www.nndc.bnl.gov/nudat2/ acceptable? -- Veggies (talk) 13:46, 22 January 2018 (UTC)
- @Veggies: It's probably mostly okay, but in some places it would be better to have confirmation from another source (e.g. whatever the most recent NUBASE is); for one thing, the superheavy region still has a lot of predicted data (e.g. 291,292Ts), and for some reason does not include 294Og. Double sharp (talk) 00:45, 6 February 2018 (UTC)
- I'll try to work on the graphic in the coming weeks, but where is the best source for info? Is http://www.nndc.bnl.gov/nudat2/ acceptable? -- Veggies (talk) 13:46, 22 January 2018 (UTC)
- I had to compute new versions of such charts (Segre' charts) for the article Valley of stability. Without even examining the objections above, I can say that they are not easy to plot. Nuclides often have several decay modes, etc., there are different nuclide spins, etc., etc. and all of these complications have to be brushed aside to make a simplistic graph (that is still worthwhile). I am also not that sure that the various data bases for these charts, from which they are plotted, are altogether in agreement. Ideally, one would have to go through each nuclide by hand, block by block, and examine the various decay modes and make an informed decision as to how best to represent the decay mode for each nuclide. Instead, some (imperfect) algorithm must be implemented to do it automatically, with mixed results. The point is just that such charts are not that easy, so I suspect we must live with imperfections. (And I'm not sure how well my own figures will stand up to your scrutiny!) Bdushaw (talk) 12:56, 21 January 2018 (UTC)
- If the new map File:NuclideMap stitched.png is added, it should be redrawn with axes labelled and numerical values added as in the old map shown here. Dirac66 (talk) 11:51, 21 January 2018 (UTC)
Error mentioning Isobar in opening paragraph[edit]
The opening paragraph has this phrase: transforming the original nuclide to its isobar, as if a nuclide has only a single Isobar. Nuclides often have several isobars. I'm changing it to an isobar. —MiguelMunoz (talk) 22:08, 13 November 2019 (UTC)
Problems with style?[edit]
A banner at the moment suggests the article has style problems, particularly with radicals. However, I don't know what that means, and I don't think the article has any radicals at all. Anybody know what the style problem is? Bdushaw (talk) 19:43, 20 August 2022 (UTC)
- There is an explanation at MOS:RADICAL, but if the criteria have now been met then delete the banner. Alternatively just check in with User:Beland who placed the banner there. NealeWellington (talk) 08:52, 13 May 2023 (UTC)
- C-Class vital articles
- Wikipedia level-5 vital articles
- Wikipedia vital articles in Physical sciences
- C-Class level-5 vital articles
- Wikipedia level-5 vital articles in Physical sciences
- C-Class vital articles in Physical sciences
- C-Class physics articles
- High-importance physics articles
- C-Class physics articles of High-importance




