Talk:Hemoglobin
| This It is of interest to the following WikiProjects: |
|||||||||||||||||||||||||||
| |||||||||||||||||||||||||||
Wiki Education Foundation-supported course assignment[edit]
![]() This article was the subject of a Wiki Education Foundation-supported course assignment, between 6 October 2020 and 16 December 2020. Further details are available on the course page. Peer reviewers: AshleyJennings.
This article was the subject of a Wiki Education Foundation-supported course assignment, between 6 October 2020 and 16 December 2020. Further details are available on the course page. Peer reviewers: AshleyJennings.
Above undated message substituted from Template:Dashboard.wikiedu.org assignment by PrimeBOT (talk) 22:17, 17 January 2022 (UTC)
Wiki Education Foundation-supported course assignment[edit]
![]() This article was the subject of a Wiki Education Foundation-supported course assignment, between 12 August 2020 and 25 November 2020. Further details are available on the course page. Student editor(s): JoyousMuse.
This article was the subject of a Wiki Education Foundation-supported course assignment, between 12 August 2020 and 25 November 2020. Further details are available on the course page. Student editor(s): JoyousMuse.
Above undated message substituted from Template:Dashboard.wikiedu.org assignment by PrimeBOT (talk) 23:19, 16 January 2022 (UTC)
Cooperative Binding[edit]
It might be helpful to include some mention of the T and R states in this section. It does address the change in conformation, but specificity is always preferred. Dykeszr (talk) 14:37, 6 April 2017 (UTC)Dykeszr
The graphic shows the change from T to R state, which is really informative so I believe that inclusion of the specific states and some information on them would benefit the page AshleyJennings (talk) 18:13, 14 October 2020 (UTC)
Article structure and HB chemical representation[edit]
is it better to have structure and synthesis together? The edit about chemical formula of Hb is not accurate. Does it refer to HbA, HbA2, HbF which have varying numbers of amino acids? " Hemoglobin is chemically represented by (C2952H4664N812O832S8Fe4). "
- The sum formula is precisely what I was looking for when I visited this article. I strongly suggest to include it into the article in the section "structure". Is there a reference for this formula? --Yen Zotto (talk) 09:23, 16 May 2012 (UTC)
As I see it, the empirical formula C738H1166N812O203S2Fe is not correct. This because it doesn't add up if you multiply it by four (which I assume the original formula was divided with).Yfé (talk) 09:37, 1 December 2008 (UTC)
Decay Time[edit]
How long does it take for hemoglobin to decay if blood falls out of a slaughtered animal onto the ground (or someplace not in the animal)? ![]() Chiss Boy 12:34, 4 March 2007 (UTC)
Chiss Boy 12:34, 4 March 2007 (UTC)
- Google it? MaximusEditor (talk) 07:14, 1 October 2020 (UTC)
4 chains vs. 2 chains[edit]
I read some time ago that while in "higher" animals including humans, 2 strands of alpha-hemoglobin combine with 2 strands of beta-hemoglobin, in "lesser" animals, the hemoglobin consists only of 2 alpha-strands (enclosing, as usual, an iron atom). Unfortunately, I had no luck finding more info on the Internet - could somebody who knows about this please elaborate? Aragorn2 11:50, 23 Oct 2004 (UTC)
If you go "low" enough, hemoglobin acts on nitrous oxide rather than oxygen [1]. A little "higher" and animals have large molecular weight hemoglobins that are carried in the plasma. Move on up to birds and mammals, and there's small molecular weight hemoglobins that are packaged in red blood cells [2]. The respiratory protein of "lower" animals certainly isn't always simpler (snail hemocyanin, for example, has 10 subunits). I don't know of any animals that have only two alpha-strands as a functional hemoglobin, and alpha-beta dimers are ineffective oxygen carriers, so something's been lost since your read it, or the missing info is in a veterinary source somewhere. - Nunh-huh 14:08, 23 Oct 2004 (UTC)
Thanks for the links. I had read about the worm before, but from this text, it's not obvious whether the worm has dimer or tetramer (or even monomer?) hemoglobin. After another Google search, I found this:
and a possible scenario for hemoglobin evolution on this page:
So the lamprey has alpha-alpha-dimer hemoglobin which splits apart when oxygenated, which is what I was referring to.
Got it now.. looks like:
- hagfish: monomeric hemoglobin in most species, some have hgb that is dimeric when deoxygenated and monomeric when oxygenated
- lamprey: monomeric when oxygenated, dimeric or tetrameric when deoxygenated
- sharks: dimeric when oxygenated (α1β1); tetrameric when deoxygenated
- vertebrates: α2β2 dimers
That was a very interesting question! we ought to put a chart or a synopsis in the article. - Nunh-huh 03:52, 24 Oct 2004 (UTC)
There are variety of hemoglobin predecessors, many of which have different names. Such as Erythrocruorin in worms and myoglobin which is a monomer and has no arrogating ability. Erythrocruorin should get an article for it self, I can easily scrounge up enough information and generate image of the protein from pubmed, but I lack the time.--BerserkerBen 22:58, 31 Jan 2005 (UTC)
Human Hemoglobin Levels[edit]
I don't know much about the chemistry of hemoglobin and the like, but a section detailing how and why hemoglobin levels are measured in humans. A table or something illustrating the distribution and ideal hemoglobin level would also be great. My doctor just called me and all he said was "14,7" and I have absolutly no clue what that means. Google will answer me in minutes, but still, I was hoping Wikipedia would have it. --Crucible Guardian 6 July 2005 01:29 (UTC)
- The "Diagnostic uses" section of the article puts 14.7 in the normal range.--MartinezMD (talk) 23:22, 31 May 2009 (UTC)
- Me too! I just found out mine is 17.4 which is higher than normal, but again this article doesn't explain the signifance of higher levels of hemoglobin.--77.224.22.177 (talk) 20:59, 31 May 2009 (UTC)
- The article is focused on the hemoglobin molecule, which is a fairly technical subject. It's also not a self-help article. You may find more information under the term Polycythemia which is more of a clinical term and possibly more helpful to you.--MartinezMD (talk) 23:22, 31 May 2009 (UTC)
hemoglobin and aging[edit]
Sometime in the past I read that in humans, oxygen transport decreases by 1% per year after the age of 20, which I can believe, being now 65. Is there information on the aging process and oxygen transport that could be summarized in a short paragraph? — Preceding unsigned comment added by 71.141.160.19 (talk) 04:59, 2005 December 2 (UTC)
- If someone supplies the references, yes (see WP:CITE). A quick Google will probably yield some results. JFW | T@lk 08:51, 2 December 2005 (UTC)
Consistency[edit]
This article goes back and forth between hemoglobin and haemoglobin. Pick one and stick with it, but mention both in the first sentence. I believe the hemoglobin spelling is more universal throughout medical literature, but I don't know about common usage outside the US. Hichris 17:39, 16 December 2005 (UTC)
- It was pretty uniform at "hemoglobin" until this was changed [3]. My literature might have been american, but I think hemoglobin is the one to choose. / Habj 03:46, 17 December 2005 (UTC)
- I would prefer hæmoglobin! That is the traditional spelling. Failing that, then haemoglobin. It's only in North America that the "simplified" version is used. EuroSong talk 09:30, 2 November 2006 (UTC)
- I guess haemoglobin would be better as it is the traditional spellingVik4989 06:25, 5 August 2007 (UTC)
- You only went back a month. Had you gone back to January, you would see the Haemoglobin spelling the article first began with was still "pretty uniform". 2001:8004:5160:17E8:B7F4:593E:152D:6CFC (talk) 11:58, 9 May 2023 (UTC)
- I would prefer hæmoglobin! That is the traditional spelling. Failing that, then haemoglobin. It's only in North America that the "simplified" version is used. EuroSong talk 09:30, 2 November 2006 (UTC)
Yeah we should really be using haemoglobin. —Preceding unsigned comment added by 194.81.199.59 (talk) 16:44, 11 February 2008 (UTC)
Hemoglobin is the spelling far more commonly used in scientific literature. Searching Medline turns up ~76,000 hits for "hemoglobin" and under 20,000 for "haemoglobin." —Preceding unsigned comment added by 128.237.250.128 (talk) 20:32, 24 April 2008 (UTC)
The Lancet gives 18 results for hemoglobin, and 1596 results for haemoglobin... —Preceding unsigned comment added by 86.150.188.15 (talk) 14:35, 23 January 2011 (UTC)
As the World Health Organisation uses the spelling "haemoglobin" would it not be more correct to use that spelling? —Preceding unsigned comment added by 78.144.149.123 (talk) 21:33, 16 February 2011 (UTC)
I tried a redirect but some automated process is not letting me to add the content to the Haemoglobin part. Some one fix it! 61.3.151.103 (talk) 09:52, 29 July 2018 (UTC)
Feature article anyone?[edit]
Anyone think this artilce shoukld be nominated as a feature article? If not why?--BerserkerBen 07:31, 31 December 2005 (UTC)
- It is not ready. Saravask 20:01, 31 December 2005 (UTC)
- What needs to be done to make it ready? --BerserkerBen 20:06, 17 January 2006 (UTC)
I can think of some points. Firstly, the spelling is inconsistent (BE/AE). There is no history section; we don't learn who discovered it, when it was first sentenced, linked and cloned, why Linus Pauling won the Nobel Prize in relation to it, why the Bohr effect is called like that, etc etc. In short, more context. Classic references are very useful. JFW | T@lk 21:28, 17 January 2006 (UTC)
- Great can we list those things up on top of the discussion board as things needed for improvemnt?--BerserkerBen 23:59, 17 January 2006 (UTC)
- Personally, as a layman, I found this article unhelpful and too technical. I came to it looking for an interpretation of my blood test results and still feel uninformed as to the signifance of my hemoglobin levels of 17.4 --77.224.22.177 (talk) 21:01, 31 May 2009 (UTC)
opening part of article not clear[edit]
"Hemoglobin or haemoglobin (frequently abbreviated as Hb or Hgb) is the iron-containing oxygen-transport metalloprotein in the red cells of the blood in mammals and other animals; in mammals the protein makes up about 97% of the red cell’s dry content, and around 35%, including water."
35% .. ??
Jdruiter 23:32, 28 January 2007 (UTC)
I was in the ER two times yesterday with my sister for nausea.Her hemoglobin was 7.9.today she still has a bad lingering headache. Could this headache be caused from her low hemoglobin? She has been sick for years. She's has really bad migraines, has had her gallbladder and half her intestines taken out,and she can't digest her food which makes her throw up.she's had to have a blood transfusion 5 months ago, because her hemoglobin was 6.1.Can anyone answer my question about if low hemoglobin can cause her to have this lingering headache?(she only weighs 80 lbs and is 56 yrs.old.)(she said this headache is not like her migraines.)
Thank you,
Sincerley
Judy Vasilis
2 images for R to T transformation?[edit]
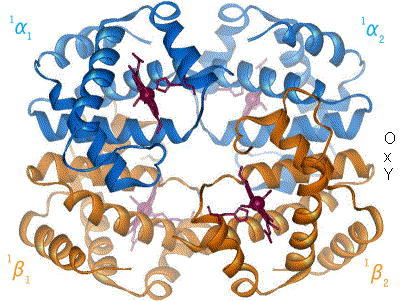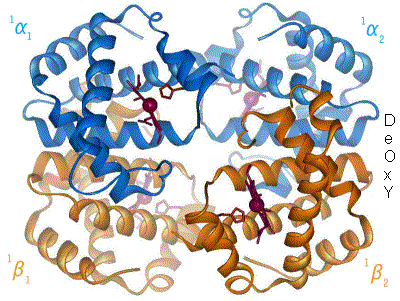
I have undone a recent addition of a second image to show the R to T transformation. [4] The reasoning is that, although the second image does show all 4 chains and you could argue that two images are better than one, the text was being crushed up along the left hand side (as the second image is so big) and the sigmoid curve image (which is already some distance from the text referring to it) was being pushed further down the page. If someone who is better at image placement than I am can resolve these difficulties I would have no problem with the 2 images. Thanks. Mmoneypenny 21:55, 28 April 2007 (UTC)
- Remember, we're now all being asked to take out the "px" setting in images, and allow our personal readers to set that for us, so all images in an article come out the same size. If you've got yours set too high (look at your "my preferences") like 300 px vs. the recommended 180, then a lot of pics which support 300 will crowd your screen more than you'd like. Also, the images that don't have enough detail to be 300 px wide or long will come out odd size, and small by comparison with 300. But fix that on YOUR end, not in the wiki.
I'll do my best to make two pics of hemoglobin at 180px come out without shoving the sigmoid curve too far down. But we really need two pics of hemoglobin motion at least as much as the sigmoid curve. SBHarris 22:09, 28 April 2007 (UTC)
- Remember, we're now all being asked to take out the "px" setting in images, and allow our personal readers to set that for us, so all images in an article come out the same size. If you've got yours set too high (look at your "my preferences") like 300 px vs. the recommended 180, then a lot of pics which support 300 will crowd your screen more than you'd like. Also, the images that don't have enough detail to be 300 px wide or long will come out odd size, and small by comparison with 300. But fix that on YOUR end, not in the wiki.
- Later. Okay, I've done my best to make it all fit, by putting the moving O2 binding pics side by side. Constraints are that the one image (the old one) requires the command "frame" to move at all. And I cannot get it to any size other than 300 px, no matter what I order. The other image is perfectly well behaved. I moved the sigmoid curve up, and reduced its size do it fits better along side the text it references. I don't know what 180px readers will do to this, for those people who insist on removing all "px" image commands. But this is a case where I think it's justified to leave all the extra commands in. Wiki policy allows this, where you just can't get it to look good any other way. SBHarris 22:43, 28 April 2007 (UTC)
- I appreciate that we are removing the px settings. I also appreciate that we can set the thumbnail size in our preferences (this is set at 180 for me) but I hope you appreciate that a framed image will always be displayed at its full size and the px command will not affect it, so this is not something I can change at my end. (Regardless of this and slightly unrelated, most people browse wikipedia without being logged in and they cannot set a preference.) Lastly, do we really really need two pics of hemoglobin motion? IMO we are trying to make people appreciate that there is a motion (as explained in the text) and the first pic shows this quite well, the second pic adds the other 2 chains but removes the oxygen... Surely if people read the text and appreciate that Hb consists of four chains and (as the first pic states) we are only showing one, that is enough? I see that the two images are now at the top of the subheading with some text shoved in between them, this still IMO looks bad. The result is an extra pic but a messy article. When I've got a bit more time I'll see if I can play around with the pics a bit more. All the best.Mmoneypenny 08:37, 29 April 2007 (UTC)
- In my reader, when I set the one nonframe illustration to 300px, there's no room for any text, so it looks pretty good. I had no idea others were having a problem.
I haven't found a way to keep text out of a section that you only want to put illustration boxes in, no matter the size. I finally put in enough equalsign spacer to keep text out of there no matter what the illustration size is. What do you think of the look now?
The main reason I'm trying to keep the old illustration in is not because I made it or had anything to do with it. But it shows the motion of the porphyrin ring-- how it moves to react with the O2 and tugs on its peptide chain by doing so, far better than the other. I can almost see all the details of how the molecule works here, and would far rather remove the latest version, if I had to pick one. SBHarris 05:38, 30 April 2007 (UTC)
- I asked for help using the {{helpme}}tag on my talk page and another user has modified the paragraph. Okay, I know now the two illustrations are distant but... how about rewriting the paragraph and the image blurbs to match the new setup AND we can place the image you prefer at the top and the other one further down. Would do this myself but am slightly busy at the mo (aren't we all?) Whaddya think?Mmoneypenny 20:35, 30 April 2007 (UTC)
- Unfortunately, the "help" didn't help at all. Either of us could simply have done what was done, which was stick one of the binding pics way down in the article where it didnt' have anything to do with the text. But they both need to be up near each other, and still give the sigmoid curve some room to be were it needs to be. And unfortunately the sigmoid discussion needs to be right after the binding discussion, in order to make sense. So the problem is not solved. SBHarris 21:48, 30 April 2007 (UTC)
- I asked for help using the {{helpme}}tag on my talk page and another user has modified the paragraph. Okay, I know now the two illustrations are distant but... how about rewriting the paragraph and the image blurbs to match the new setup AND we can place the image you prefer at the top and the other one further down. Would do this myself but am slightly busy at the mo (aren't we all?) Whaddya think?Mmoneypenny 20:35, 30 April 2007 (UTC)
- In my reader, when I set the one nonframe illustration to 300px, there's no room for any text, so it looks pretty good. I had no idea others were having a problem.
(cough)I was the one that made taht animation if you need my help or anthing? (cough) --BerserkerBen 23:38, 30 April 2007 (UTC)
- We need animations which don't force the reader to see them at 300px because they've been done via FRAME. I don't know if that's possible, but it must be, because the first animation does it. But I can't make the second one do it, and still move. The problem is that having one at 300px and the other at the default size of whatever the viewer's reader is, is bound to screw up the text, if the two are near each othr. Which it would be nice if they were. But nobody's been able to get them side by side and keep everybody happy. SBHarris 02:56, 3 May 2007 (UTC)
- Well I guess it some flaw with my gif animator if you want the orginal frames so you can animate it please ask.--BerserkerBen 03:49, 3 May 2007 (UTC)
The animated gif is very nice for demonstrating not only that the chains move relative to each other, but also that while internal conformation changes occur in the alpha chains, the beta chains move pretty much as rigid bodies. Unfortunately the helix definitions in the beta chain on the right have been made differently in the two states, so one helix dissolves completely and another loses one turn, in going from deoxy to oxy form. The picture should be remade with the same helix definitions in oxy as in deoxy.Eaberry (talk) 15:18, 12 April 2011 (UTC)
I have been studying hemoglobin's structure and many biochemistry textbooks are contradictory on whether identical (alpha or beta) subunits are adjacent or diagonal. The first image of this article has them labeled diagonally whereas this image has them labeled adjacent. Frustrated, I visited what I believe is the most authoritative site: the PDB (Protein Data Bank) which catalogs the x-ray crystal or NMR structures determined by research groups and found that the correct labeling is diagonal (as in the top diagram of this article). Thought you might like to know this. Thanks. 126lys (talk) 19:09, 4 January 2011 (UTC)
- It depends on the orientation- both figures were made from the pdb files and are correct. Notice the shading- In the first figure the alpha chains are near and the beta chains far from the viewer. Now rotate the molecule 90 degrees about a horizontal axis (top pushing back) and the alpha chains will be side-by side on top, the beta chains will be side by side on the bottom as in the second (animated) picture- but only in projection, actually the left alpha is close and the right is far; and vice versa for the beta's. The four chains occupy 4 vertices of a tetrahedron, and each is adjacent to all three other chains.Eaberry (talk) 15:18, 12 April 2011 (UTC)
Oxidation state[edit]
The section about the oxidation state of iron in oxyhaemoglobin is wrong. It is now known that it is low-spin Fe(II). See Greenwood and Earshaw, Chemistry of the Elements 2nd edition, pp 1099-1100. The key point is that in deoxy-Hb the high-spin Fe(II) is too large to enter the plane of the porphirin ring (Figure 25.7) but when oxygen binds the low-spin Fe(II) enters the plane as it is small enough. The paramagnetism of O2 is destroyed because the Fe-O2 angle is 120o, so the pi-star orbitals are no longer doubly degenerate.
I'm not a biochemist, so I hesitate to edit the article myself.Petergans 09:56, 11 May 2007 (UTC)
- I made the same mistake in originally fleshing out this section. Apparently the old classic crystal field theory view of the iron pi-star orbitals being forced to give up their electrons into low spin pairs when O2 enters the picture, is wrong. Fe(III) when present in free hemoglobin won't bind oxygen, but oddly enough X-ray spectrocopy and IR show that iron in oxygenated hemoglobin actually is Fe(III), and the O2 bond had elongated enough that it's really a bond with order 3/2, i.e. it is superoxide .O2-. So the (surprising) fact is that O2 binding to hemoglobin involves a (temporary) partial oxidation of iron to Fe(III) and reduction of O2 to .O2-. Basically about one electron gets temporarily transferred in a charge-transfer complex (of course you realized that electrons often don't get transfered exactly, but only approximately, since these bonds have some covalent character). Anyway, that's the modern picture. SBHarris 18:40, 11 February 2008 (UTC)
-SBHarris, do you have reference for that? —Preceding unsigned comment added by 216.80.44.45 (talk) 02:06, 24 October 2008 (UTC)
- Yes, which I'll add: http://www.ul.ie/~childsp/CinA/Issue65/TOC28_Haemoglobin.htm. Basically, all the evidence points to oxyhemoglobin being a complex of low-spin Fe(III) and superoxide. The oxygen bond order is 1.6 by IR, meaning it's been nearly reduced to superoxide, and the whole complex is diamagnetic (no net spin) which can only happen if the single unpaired electron on O2.- pairs with the single unpaired electron in low spin Fe(III) by a long distance (anti)ferromagnetic interaction, giving the whole complex no net spin. The standard idea of a triplet neutral O2 (paramagnetic) paired with low spin Fe(II) (diamagnetic) would produce a net paramagnetic HbO2, and HbO2 isn't paramagnetic. To get a diamagnetic HbO2 with low-spin Fe(II) you have to have diamagnetic O2, which means singlet oxygen. This higher energy solution is apparently not chosen by nature. Extraction of an additional electron from iron allows for a smaller Fe atom, and that allows it back into the plane of the porphyrin ring, which needs to happen for the allosteric interaction. I'll do some more explaining in the article. SBHarris 04:00, 28 October 2008 (UTC)
Can we get a primary reference for the claim that IR frequencies change with the state of O2? For example is this Raman data or IR data? IR is usually not ideal for measuring absorption of diatomic molecules (though this may not be a problem for O2 coordinated with the hemoglobin).Jesse Greener (talk) 19:40, 2 April 2010 (UTC)
hemoglobin or haemoglobin[edit]
Is it called hemoglobin or haemoglobin. The title says hemoglobin but in the opening line of the article it says haemoglobin. Which one should be changed? Ziphon 02:47, 29 May 2007 (UTC)
- Hi. There's no consensus on the "correct" spelling. However, we should use 1 spelling throughout the entire article for consistency, and mention alternate spellings only once in the article's introduction. Greets, A. Rad 16:29, 30 May 2007 (UTC)
- Considering that Funk spelled the original (in German) word "Hämoglobin" in 1851, I'd suggest that has seniority. The "Haemo" spelling is clearly a more faithful transliteration than "Hemo", both are widely used so we need a redirect anyhow. Why not use the original for the title and make both transliterations redirects?LeadSongDog (talk) 20:04, 18 April 2008 (UTC)
- The remainder of the article employs the American flavor of English (personally, I prefer grape, but I'm rather odd), so tradition and WP:ENGVAR agree that we should stick with that unless somebody converts the entire article to another flavor, an act which is usually considered to be very bad form. It seems to me, then, that hemoglobin (and fetus) are most appropriate. – ClockworkSoul 21:36, 18 April 2008 (UTC)
The original article was written in British English, and according to wikipedia rules, any form of english that is widely accepted is acceptable, so long that this was the original form the article took. Applying this, given the original article (tracking history back as far as i can) has the spelling 'haemoglobin', I move for this to be reinstated. 86.7.204.43 (talk) 21:30, 30 August 2008 (UTC)
- True, but the article was created at the American spelling, Hemoglobin, and a spelling change would require a move. It's hard to say whether it's the article creator's choice of location or primary spelling method within the article that should be honored (honoured?) first. It's kind of an odd situation, and I'm inclined to say leave well enough alone. -- Vary | Talk 18:42, 31 August 2008 (UTC)
- Hi everyone. I propose that this discussion be continued. According to the Wikipedia Manual of Style - Consistency Within Articles (see [5]) - "Each article should consistently use the same conventions of spelling, grammar, and punctuation. For example, these should not be used in the same article: center and centre; insofar and in so far; em dash and spaced en dash". I believe that adequately substantiates a conversion of the spellings to a more consistent form, ie American or British. (<That was a mouthful of a sentence, @_@, sorry about that). Anyway, the real question is to which style of spelling should be used. I tried to find an answer via the Wikipedia Manual of Style (MoS), and this is what I got... "In the early stages of writing an article, the variety chosen by the first major contributor to the article should be used" - this would solve most disputes, but, it seems the original conributor/creator used both the American "Hemoglobin" as the title, and the British "Haemoglobin" for the rest of the article (see [6]). From how I interpreted the above discussion, this was considered a dead-end and was simply be left as it was. I disagree with this "If we can't find the answer -then leave it alone" logic and would like to refer to another part of the MoS Consistency Within Articles ([7])which states "If an article has evolved using predominantly one variety, the whole article should conform to that variety". Considering the ambiguity concerning the choice of spelling by the original author, I would normally have proposed that we take note of the above quotation from MoS and use the spelling in which the article evolved in. This would be the "Haemoglobin" spelling over the "hemoglobin" spelling considering that other than in the title, all of the first 5 major edits used the British spelling (see([8]). BUT. The fact that the title of the article was originally "Hemoglobin" is still a very important factor, not one that can be ignored. Sorry for regurgitating some information that already seems to have been added, but, a consensus really needs to be established as which spelling soon because currently it looks quite silly spelling the title one way only to contradict itself within its first separate section (Research History - It even uses both types of spelling within the same section). I'm not going to change anything without agreement first, so no one worry about me going edit-crazy :D. I'm looking forward to further discussion about this. Sincerely and Respectfully, Gilly of III (talk) 03:12, 6 October 2009 (UTC)
- You can't tell from the page history what the name of the article was back then, but the edit summary at the redirect suggests that you are correct in assuming that the text and the page name originally did not match.
- The MoS doesn't actually require any particular solution; its primary purpose is to avoid edit warring. "Whatever was done first is fine" is just a standard default rule to prevent edit warring without having to universally favor one style over another. If editors here decided to clean up the article this way or that way, then they are allowed to do so, even if their decision isn't precisely what the "first" or "first major" editor did. WhatamIdoing (talk) 21:52, 6 October 2009 (UTC)
- Show me *one* article in the current literature, which uses the spelling haemoglobin. Hemoglobin is the accepted way of spelling it. Even Max Perutz chose to spell it hemoglobin at the end of his careeer; e.g. http://www.ncbi.nlm.nih.gov/pubmed/9646860. Carstensen (talk) 15:26, 5 December 2011 (UTC)
- There are dozens of examples; this search should show them for you. They include PMID 19712775, PMID 20061360 and so on. Many journals (especially American journals) insist on their local spelling, so often the author has no "choice" which spelling to use. Annual Reviews are based in the USA, so I doubt this was Perutz's choice. --Stemonitis (talk) 16:43, 5 December 2011 (UTC)
- I favour hæmoglobin, because that spelling most faithfully represents the actual pronunciation, and had not been dumbed down for people who don't know how to type æ. EuroSong talk 10:27, 7 October 2014 (UTC)
Globin genes[edit]
I come from more of a genetics background and knew hemoglobin in the context of the alpha globin locus, the beta globin locus and locus control region, the genes, etc. It took me forever to get my bearings here because "variants" refer to different combinations of protein subunits, not different protein subunits themselves. I now realize that α,β,etc. are the protein subunits, but I don't think this was explained very well and the protein subunits themselves definitely aren't talked about much. For now I'll just add links under "See also" to the genes, since I'm not sure on a good consistent nomenclature for the article. Forluvoft 06:52, 3 November 2007 (UTC)
- Wups, "variants" is used in BOTH senses: not only to different tetramers, but also (and mainly) to the many, many polymorphisms in the protein chains themselves (like sickle cell, but producing no clinical problems, even when homozygous). This needs a fix, to differentiate. We at one time had an actual article on Hemoglobin variants which I started, and which somebody took down and redirected to hemoglobin! I put it up at first for this very reason. Damn deletionists. This is a good example of how they harm the encyclopedia. There certainly isn't room enough in this article to discuss all of these sequence variants, only to discuss the tetramer variants. The gene variant article subarticle which had't grown up yet, and was killed in its cradle. Arghh. If you complain about this kind of thing, some admin who isn't interested in the subject will say that "wikipedia isn't the humane society for lost information." Well, that's wrong, or should be. SBHarris 18:06, 11 February 2008 (UTC)
Genetics and synthesis[edit]
The genetics of haemoglobin is quite complex and changes with the age of the embryo and child. None of the complexity is shown here. Please see the article on Fetal haemoglobin; http://en.wikipedia.org/wiki/Fetal_hemoglobin for a start. The genetics are also very interesting as the gene is really more of a region than a locus. Most of the sequence is coded forward but some is coded in reverse direction. Sequences are moved around as transcribed into mRNA and then other sequences are moved and deleted in the protein assembly. This synthesis is missing. 198.103.184.76 (talk) —Preceding undated comment added 15:39, 11 March 2013 (UTC)
Too much?[edit]
The "role in disease" section lists several diseases of "too little" or "broken" hemoglobin. What is it when you have too much hemoglobin? Polycythemia? Something else? WhatamIdoing (talk) 03:55, 22 August 2008 (UTC)
- In practice, polycythemia is the only way this happens. It's not so much the having of too much hemoglobin that is pathological, as having too much fraction of blood made of cells (packed cell volume or "hematocrit" too high). These amount to (almost) the same thing because concentration of hemoglobin in cells can't vary much (it can been lower than normal, but not much higher, and cannot vary enough to affect the major problem of too many cells). In theory your total cell mass per volume could be high enough to make you ill (clots) but due to giant cells with low hemoglobin, everything but packed cell volume would be normal. However, in practice you don't see this. And you can't see high hemoglobins without also high PCVs because of the fact that hemoglobin is already at near the max concentration in normal cells anyway, so the only way to increase it in blood significantly is to incease the total cell-volume fraction in blood. Sorry that's not clearer, but it's written on the fly. SBHarris 21:38, 30 August 2008 (UTC)
- Thanks. I added it and hope we can find a good source for it. WhatamIdoing (talk) 02:44, 31 August 2008 (UTC)
Reference range[edit]
I do not know much about the chemistry of hemoglobin and the like, but a section detailing how and why hemoglobin levels are measured in humans. A table or something illustrating the distribution and ideal hemoglobin level would also be great. My doctor just called me and all he said was 7 and I have absolutly no clue what that means. Google will answer me in minutes, but still, I was hoping Wikipedia would have it. --Crucible Guardian 6 July 2005 01:29 (UTC) —Preceding unsigned comment added by Ohiohealthquiz (talk • contribs)
- You'll find your answer at this page, but it could be added to this article (or a link provided to the other one) if someone wanted to do that. WhatamIdoing (talk) 03:01, 31 December 2008 (UTC)
Discovery?[edit]
I don't know about this edit. If we've got a source that says 1851, then why are we replacing it with one that asserts a 1940 discovery? WhatamIdoing (talk) 20:11, 8 January 2009 (UTC)
- It depends what you mean by discovery. Observation? Isolation? Structure? — Preceding unsigned comment added by Carstensen (talk • contribs) 15:30, 5 December 2011 (UTC)
From the paragraph headed Research History:
- In 1825 J. F. Engelhard discovered that the ratio of iron to protein is identical in the hemoglobins of several species.
Not a claim of discovery, but a fairly strong implication that someone else had already discovered hæmoglobin; granted, Engelhard did not use that term but instead called it vera materia sanguini purpureum colorem.
I found this on an American Chemical Society webpage (https://www.acs.org/content/acs/en/molecule-of-the-week/archive/h/hemoglobin.html):
Here are some significant events in the history of hemoglobin discovery:
- 1825: J. F. Engelhard shows that the Fe/protein ratio is the same in several species’ hemoglobin.
- 1840: F. L. Hünefeld discovers that hemoglobin transports oxygen.
I think that makes "protein" a likely typo for "property of" in the bit about Hünefeld, but I do not have access to the primary sources. Regardless, for Engelhard to have come up with such a close (and, at the time, astonishing) estimate for the molecular mass of hæmoglobin deserves a much wider currency.
I'm going to make the edit and see what turns up. Moletrouser (talk) 10:21, 23 September 2018 (UTC)
Who is the audience for this article?[edit]
This is a very interesting article, with great information and graphics. However, I must comment that this page seems to require too much prior knowledge of biochemistry or medicine from the general reader, the presumed audience/user. For instance, why the presumption that the reader knows what an "erythrocyte" is? It would be arrogant to say, "Well, they can just look it up." That is poor editing if the intended audience is not limited to those with a chemical or biological major in college or medical school training. Why not at least define as "red blood cells"? Also, the inconsistent spelling of hemoglobin in the very heavy section "Presence in nonerythroid cells" is distracting and possibly confusing, if one didn`t know that that was all it was. Again, this article is impressive, esp. as it is "Scot-free"! (non-PC comment, LOL) Jack B108 (talk) 02:03, 15 January 2010 (UTC)
- We've fixed as many of the direct problems you refer to as I can find, but your general question of audience is harder. WP has no official answer to it! Usually the "lowest" level written at is about high-school level, and (ideally) it progresses in demands on the reader from there, as articles move through various levels suggested by going through subarticles. The clearest expression of this in in the math articles. The problem with doing this in science articles is that if the "entry level" article is not long enough, there is no pressure to spin off subarticles per WP:SS, and then the material in the entry article can become quite technical because no re-write split to higher/lower tech levels has been forced. That is what is happening here. Eventually some of the more complex material will be spun on into subarticles and the rewritten section left behind will be written at a lower education level. Meanwhile, things are somewhat uneven in the primary article.
If you find places where you find the techical level unevenness unusually bad, I suggest you fix it by adding introductory explanatory material to get from the level you feel comfortable at, to that of the present text. If doing so increases the length of this article so much that further spin-offs become necessary, that's fine! It will then trigger the WP:SS mechanism. So this is a natural mechanism of growth, and a self-regulating one when it comes to technical level. It's just that this sort of evolution occurs with less gradualism and uniformity than many people who are used to "non-grown" encyclopedias are comfortable with. SBHarris 02:57, 15 January 2010 (UTC)
- IMO it's often useful for important sections in technical articles to have a "lead" of their own, that gives a plain-language summary before getting into the sometimes eye-glazing details. We'd like to have things written well enough that most teenagers could at least figure out kind of what each section is about, even if they don't understand anything else in the section. WhatamIdoing (talk) 05:10, 15 January 2010 (UTC)
- Absolutely! Feel free to add intro sentences or a paragraph to technical sections as necessary. Small intros at the general audience level never hurt (even knowlegable people who don't need them often appreciate them, as a break from lessen relentless technicality), while at the same time, they can be essential for less knowlegeable people who otherwise would be left completely in the dark without them. So again, I heartily agree with the idea of adding clear-language lead-ins to all tech material. SBHarris 06:46, 15 January 2010 (UTC)
- Ok, thanks. It already looks better (I was maybe having 'slow-brain syndrome' when I first read "erythocyte" here and wondered, "now what the heck is that?" This is a very important article, for a landmark protein, and it is great that anyone with the Internet can read it. Jack B108 (talk) 00:29, 17 January 2010 (UTC)
Iron's oxidation state[edit]
This section gives a good account of the historical facts, but the conclusion is now known to be wrong - see Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. p. 1099. Hb-O2 is a complex of low-spin iron(II) with the oxygen molecule. The angle Fe-O-O is not 180°. The non-linearity destroys the degeneracy of the π* orbitals and so the electrons which have paralell spins in the oxygen molecule can pair up in the complex at a lower energy cost than is required to form the singlet state in the isolated oxygen molecule. This section therefore need more attention than I feel qualified to give, as I'm not expert in biochemistry. Petergans (talk) 14:12, 17 March 2010 (UTC)
- Er, what about the photoelectron spectroscopy results which indicate Fe(III) and the IR bond stretch which indicates O2 bond order ~ 1.5? A merely lowered energy for singlet oxygen doesn't explain either of these results, although singlet O2 and low spin Fe(II) was the best classical guess before these results came in. Do you have some primary paper cites of more recent vintage, instead of a text? The cites that support the Fe(III)-superoxide interpretation are pretty good and I think this is the modern accepted view http://www.ul.ie/~childsp/CinA/Issue65/TOC28_Haemoglobin.htm. But I'm willing to be convinced. SBHarris 19:49, 2 April 2010 (UTC)
- It is not just the lowered energy for singlet oxygen. Energy is also released in the change from high-spin to low-spin iron(II). In classical crystal field theory a high-spin complex would have 4 unpaired electrons and a low-spin complex would have none. Some energy is taken up in pairing the spins and some is left over and it is that energy that is left over that contributes to the driving force for the reaction. A significant factor is the geometry of the complex with Fe lying in the plane of the porphyrin ring in the low-spin state and above the plane in the high-spin state. This, in part, accounts for the difference in crystal field splitting energy. There is also the fact that the coordination number of the iron appears to increase from 5 in Hb to 6 in HbO2. Crystal field splitting energy increases with coordination number. Another point that Childs overlooks is that in both Hb and HbO2 the symmetry around Fe is C4v (square pyramidal) not Oh (octahedral). It follows that the d orbitals designated as t2g are not triply degenerate, but split into b2 (dxy) and e (dxz and dyz) symmetries.
- Childs seems to base the assignment of oxidation state on the infrared evidence, but this is not conclusive. The IR evidence can be interpreted as showing Fe-O sigma-bond formation which will result in a lowering of the O-O stretching frequency. (P. Gans, Vibrating molecules, Chapman and Hall, 1971. Chapter 9 The effects of coordination on ligand group frequencies). Arguments about the products of chemical reactions are also inconclusive since the reaction energy depends on the difference in energy between reactants and products, so the stability of the products can be the driving force for the reaction. The PE spectroscopy evidence is very indirect and also open to re-interpretation.
- The major problem with the Fe(III)-O2- hypothesis is that the electrons have to be paired up by some kind of anti-ferromagnetic coupling. If this did occur, it would be unique (to my knowledge) in the whole of the coordination chemistry of the transition metals. That is to say, it's not impossible, but very unlikely.
- You ask for more up-to-date references. A book on bioinorganic chemistry might have them. Petergans (talk) 09:33, 8 April 2010 (UTC)
Inconsistent conversion g/dL to mmol/L in Wiki-article[edit]
Conversion between g/dl is given differently in different sections (quotes A and B below).
A) Each subunit has a molecular weight of about 17,000 daltons, for a total molecular weight of the tetramer of about 68,000 daltons (64,458 g/mol)[24]. Thus, 1 g/dL = 0.1551 mmol/L.
B) Hemoglobin concentration measurement is among the most commonly performed blood tests, usually as part of a complete blood count. For example it is typically tested before or after blood donation. Results are reported in g/L, g/dL or mol/L. 1 g/dL equals about 0.6206 mmol/L
The former is intuitively right as it uses to the full weight of tetrameric haemoglobin, but it is the latter (that gives the concentration of heme-groups, 1 per subunit of approximately 16000) that is consictent with my ADVIA outputs. Can anyone give me a reason for this discrepancy?
- I agree this is confusing. If you search in google for "hemoglobin g dl to mmol l", it will only show the 0.1551 result as under A), while it is much more likely that you were looking for the clinically relevant factor of 0.6206 mentioned in B). 194.171.7.38 (talk) 16:04, 2 February 2012 (UTC)
Physiological Role of glycosylated haemoglobin[edit]
The article talks about the fact that glucose can bond to haemoglobin, and the diagnostic use of this, but doesn't mention a physiological role. Is there one? Why is haemoglobin able to combine with glucose? It would certainly help the article to either explain one, or to say that none is known, as most readers are bound to wonder about this. 144.32.126.16 (talk) 10:03, 24 September 2010 (UTC)
- It's a non-enzymatic bond, and I think the presumption is that it has no physiologic role. Glucose binds to many proteins in this manner, including albumen. See Hemoglobin A1c. SBHarris 05:45, 25 October 2010 (UTC)
Cooperative binding[edit]
It is not clear whether it is easier or more difficult for the fourth oxygen molecule to bind (as far as I know, it is more difficult) and the paragraph implies otherwise.
59.182.159.57 (talk) 03:43, 25 October 2010 (UTC)
- It is correct as written in that section. Each additional oxygen molecule that binds hemoglobin increases hemoglobin's affinity for oxygen at its remaining, unoccupied binding sites. That being the case, hemoglobin is able to load up in the high oxygen environment of the lungs very efficiently, and is also able to shed all of of its oxygen molecules in the low oxygen environment of the tissues. In other words, each oxygen that binds makes it easier for additional oxygens to bind, so it's really easy to saturate hemoglobin in the lungs. On the hand, in the tissues, as each oxygen comes off, that makes it easier for the oxygens to come off as well (efficient unloading).
- http://roselab.jhu.edu/~raj/Research/Hemo/hemo.html
- Looks like the article could be written more clearly though.
- Rytyho usa (talk) 02:44, 11 May 2012 (UTC)
Origin of the word "hemoglobin"[edit]
Based on a quick check of an online dictionary, the word "heme" was first used in 1925, well after the use of "hemoglobin". I would "guess" that the word is based on "hemo-" (blood) and "globin" (roundish protein) and was called that before heme was discovered, but I don't have a reference source to verify this. Anyone else have a specific reference for this? --Kris (talk) 17:18, 30 December 2011 (UTC)
- The Online Etymology Dictionary says: hæmoglobin, coloring matter in red blood cells, 1862, shortening of hæmatoglobin (1845), from Greek haimato-, combining form of haima (genitive haimatos) "blood" (see -emia) + globulin, a type of simple protein, from globule, formerly a word for "corpuscle of blood." For haemo- it says word-forming element meaning "blood," perhaps via Old French hemo-, Latin haemo-, from Greek haimo-, contraction of haimato-, combining form of haima "blood" (see -emia). 2001:8004:5160:17E8:B7F4:593E:152D:6CFC (talk) 11:58, 9 May 2023 (UTC)
Confusing statement about desaturation[edit]
Section: Oxygen saturation Narrative: "In general, hemoglobin can be saturated with oxygen molecules (oxyhemoglobin), or desaturated with oxygen molecules (deoxyhemoglobin)"
The phrase "desaturated with oxygen molecules" is difficult to parse. Does this simply mean "lacking in oxygen molecules", or does it mean "can be desaturated, by using oxygen molecules"? Pretty sure the former, but this is a very strange way to say so.
Also, is there no intermediate state of partially filled with oxygen molecules? — Preceding unsigned comment added by Gwideman (talk • contribs) 18:35, 1 April 2013 (UTC)
Wrong carbamino picture: Binding CO2 to iron in heme instead of Valine amino ends of alpha and beta chains of hemoglobin[edit]
The picture of heme shows the binding of CO2, but in many works is shown, that it is more probable of binding it to the amino ends of each four chains. Also the name carbamino is telling about the reaction CO2(carb) + -NH2(amino) = -NHCOOH more than Fe + CO2 = FeCO2+ or what it is? — Preceding unsigned comment added by MarekMatejak (talk • contribs) 23:19, 26 June 2013 (UTC)
Assessment comment[edit]
The comment(s) below were originally left at Talk:Hemoglobin/Comments, and are posted here for posterity. Following several discussions in past years, these subpages are now deprecated. The comments may be irrelevant or outdated; if so, please feel free to remove this section.
| Very nicely done article: should need relatively little work to advance it to FA status. – ClockworkSoul |
Last edited at 21:06, 23 October 2006 (UTC). Substituted at 17:37, 29 April 2016 (UTC)
External links modified[edit]
Hello fellow Wikipedians,
I have just modified 2 external links on Hemoglobin. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20090116021754/http://www.ul.ie/~childsp/CinA/Issue65/TOC28_Haemoglobin.htm to http://www.ul.ie/~childsp/CinA/Issue65/TOC28_Haemoglobin.htm
- Added archive https://web.archive.org/web/20080613202658/http://www.anemia.org/ to http://www.anemia.org/
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 18 January 2022).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 16:07, 31 March 2017 (UTC)
Requested move 29 July 2018[edit]
- The following is a closed discussion of a requested move. Please do not modify it. Subsequent comments should be made in a new section on the talk page. Editors desiring to contest the closing decision should consider a move review. No further edits should be made to this section.
The result of the move request was: no consensus to move the page to the proposed title at this time, per the discussion below. Dekimasuよ! 06:19, 5 August 2018 (UTC)
Hemoglobin → Haemoglobin – The preferred title has been discussed informally before, but it seems like it is time for a formal move discussion after a recent cut'n'paste move attempt. Haemoglobin is the traditional spelling, but hemoglobin is arguably now more common. See discussion above. Lithopsian (talk) 11:30, 29 July 2018 (UTC)
- The title was discussed in this section above and its worth a look before commenting here. There could be WP:ENGVAR/MOS:RETAIN issues here ("...use the variety found in the first post-stub revision that introduced an identifiable variety."). The article text was had some British English using "haemoglobin" including in the intro, but it wasn't consistent. Page moves (title moves) did not appear in early edit history summaries, but the creation date of this redirect and this edit in 2005 show that the original title was likely hemoglobin. — AjaxSmack 17:04, 29 July 2018 (UTC)
- Support dominant spelling in GBooks In ictu oculi (talk) 17:28, 29 July 2018 (UTC)
- Absolutely is not - Hemoglobin nets over 5 million results and Haemoglobin has has just 2 million. Google Ngram link below proves it further. -- Netoholic @ 11:43, 30 July 2018 (UTC)
- Since when Google search is a factor to consider in deciding those? 117.197.195.103 (talk) 13:44, 1 August 2018 (UTC)
- Absolutely is not - Hemoglobin nets over 5 million results and Haemoglobin has has just 2 million. Google Ngram link below proves it further. -- Netoholic @ 11:43, 30 July 2018 (UTC)
- Oppose Haemoglobin is not clearly dominant. Natureium (talk) 19:40, 29 July 2018 (UTC)
- Oppose current name is obviously most common per Google Ngram results and Google Scholar comparison ("Hemoglobin" at 1870k and Haemoglobin at 582k). Such WP:COMMONNAME usage means that other WP:CRITERIA are not met by this move proposal, and it would be a disservice to readers and editors. -- Netoholic @ 11:43, 30 July 2018 (UTC)
- Oppose per WP:COMMONNAME. 14:56, 30 July 2018 (UTC)
- Support I'm a med student in the US and even we don't use 'Hemoglobin'. In all our academic books, it's haemoglobin. 'Haemoglobin' is the standard spelling. 117.197.195.103 (talk) 13:44, 1 August 2018 (UTC)
- That's interesting, because based on your IP address, you're editing from India. Natureium (talk) 13:56, 1 August 2018 (UTC)
- That's about right. If I am in India, I'd be using an Indian IP only. What does this have to do with my point? 103.207.247.156 (talk) 09:27, 2 August 2018 (UTC)
- That's interesting, because based on your IP address, you're editing from India. Natureium (talk) 13:56, 1 August 2018 (UTC)
- The above discussion is preserved as an archive of a requested move. Please do not modify it. Subsequent comments should be made in a new section on this talk page or in a move review. No further edits should be made to this section.
"Iron's oxidation state in oxyhemoglobin" too much information?[edit]
Would anyone be opposed to me cleaning up and shortening the section on the oxidation state? It's all good information, but it's something of a rabbit hole that is probably not of interest (or accessible) to a general audience. I think it could all be summarized in a paragraph, pointing out the conflicting models and stating that there is disagreement about the matter. If there is general consensus to keep it the way it is, I'll shut up. WeirdNAnnoyed (talk) 03:04, 18 April 2023 (UTC)
- I deleted the section entirely: It was far too detailed, was packed with WP:SYNTH, cited few sources, and at least one of the cited sources did not mention hemoglobin at all. I simply added a brief summary of the main points, cited to an appropriate secondary source, under the "oxyhemoglobin" section above. WeirdNAnnoyed (talk) 18:36, 12 July 2023 (UTC)
Do these numbers add up?[edit]
From this article:
- A healthy human has 12 to 20 grams of hemoglobin in every 100 mL of blood ... in mammals, hemoglobin makes up about 96% of a red blood cell's dry weight (excluding water), and around 35% of the total weight (including water).
From the article blood:
- Blood accounts for 7% of the human body weight, with an average density around 1060 kg/m3
1060 kg/m3 = 106g per 100 mL
35% of 106g = 37g, well outside stated the range 12 to 20 grams
Sorry, I overlooked that 35% is for a blood cell, not blood as a whole.
Another thing, isn't it weird to give the density of blood in kg/m3? Wouldn't g/cm3 be more normal? 2A00:23C8:7B09:FA01:2165:E410:E554:7951 (talk) 18:09, 25 February 2024 (UTC)
- B-Class vital articles
- Wikipedia level-4 vital articles
- Wikipedia vital articles in Biology and health sciences
- B-Class level-4 vital articles
- Wikipedia level-4 vital articles in Biology and health sciences
- B-Class vital articles in Biology and health sciences
- B-Class Physiology articles
- Mid-importance Physiology articles
- Physiology articles about blood
- WikiProject Physiology articles
- B-Class Molecular Biology articles
- Unknown-importance Molecular Biology articles
- B-Class MCB articles
- Top-importance MCB articles
- WikiProject Molecular and Cellular Biology articles
- All WikiProject Molecular Biology articles


